Institutional Review Board
The Institutional Review Board (IRB) is a group that is tasked with safeguarding the rights and welfare of people participating in research. These participants’ rights, which include voluntarism and informed consent, are enumerated in various federal regulations and historical guidelines. The IRB must not only protect human subjects, but it must evaluate new and ongoing research with respect to science, law, ethics, and community attitudes. McLeod Health has a local IRB that reviews many research studies, but external IRB entities are also utilized when appropriate.
To learn more about IRBs and how they help protect people who participate in research, check out this video from the U.S. Department of Health and Human Services.
Membership
Diversity of IRB membership is important in order to sufficiently evaluate all types of human subject research. Membership cannot include just men or just women, nor can the Board be made up of members from only one profession. Additionally, there must be at least one member from a non-scientific area and one member who is not affiliated with the institution. Our Institutional Review Board consists of approximately 18 individuals who support McLeod’s dedication to research and patient protection. Click here for a list of our members.
Clinical Trials
McLeod Health participates in a variety of local and national research trials. Click here to learn more.
IRB Applications and Forms
These forms are also located on the McLeod Compass (Intranet).
Adverse Event/Serious Adverse Event (SAE) Form
Criteria for IRB Approval (Supplemental Form)
Humanitarian Use Device (HUD) Application
Humanitarian Use Device (HUD) Per Use Form
Notice of CIRB (NCI) Protocol Application - NEW or TRANSFER
Physician Financial Disclosure Form
IRB Meeting Dates for 2024
The Institutional Review Board will hold convened meetings on the dates listed below. Meetings will begin at 1:30 p.m. in Cancer Center Conference Room #2 (located on the 1st floor of the Cancer Center) unless communicated otherwise. Virtual meeting access is available to members and presenters through Microsoft Teams (for details, contact the IRB office at the numbers below). On meeting days, lunch will be available for IRB members starting at 1:00 p.m.
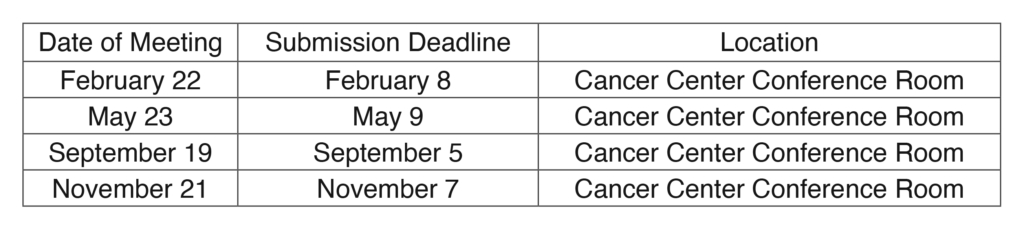
NOTE TO ALL PRINCIPAL INVESTIGATORS AND RESEARCH NURSES:
Please note the submission dates. All documentation must be electronically submitted to Toshia Jones, IRB Coordinator, by the submission deadline in order to be considered for approval at a subsequent meeting.
If you have any questions, please call the IRB Office at ext. 777-4899 (off campus, call (843) 777-4899) or email nkleckner@mcleodhealth.org.
Primary Meeting Location:
McLeod Center for Cancer Treatment and Research
1st Floor Conference Room #2
401 E, Cheves Street, Florence, SC 29506
Directions when coming from off-campus: Turn in at entrance sign 1 on the north side of Cheves Street. Park in the West Parking Garage next to the Cancer Center (take the first left after turning in at entrance sign l); or, if you prefer, you may use the free valet parking at the entrance of the Cancer Center. Walk through the automatic doors on the ground floor and continue down the concourse hall to the receptionist in front of the waterfall. Ask for the IRB meeting in Conference Room #2.
IRB Policy and Procedures
These policies are located on the McLeod PolicyStat website.
Continuing Review C-20
Children Involved in Research C-30
Definitions D-10
Expedited Review E-10
Exempt Research E-20
Expanded Access & Emergency Use of Investigational Drugs or Devices E-30
Federal-Wide Assurance (FWA) F-10
Humanitarian Use Device (HUD) Requirements H-20
IRB Fees I-10
Informed Consent I-15
IRB Human Research Duties I-20
IRB Membership and Responsibilities I-30
Meetings M-10
Member Training and Continuing Education Requirements M-15
Minutes M-20
Principal Investigator Responsibilities P-10
Problems in Human Subject Research P-30
Record Management & Retention R-10
Research Involving Pregnant Women, Human Fetuses and Neonates R-20
Signatory/Designee Authority S-10
Suspension or Termination of Research by the IRB S-20
Utilizing NCI CIRB U-10
-
McLEOD REGIONAL MEDICAL CENTER FLORENCE
843-777-2000 -
McLEOD DARLINGTON
843-777-1100 -
McLEOD DILLON
843-774-4111 -
McLEOD LORIS
843-716-7000 -
McLEOD SEACOAST
843-390-8100 -
McLEOD CHERAW
843-537-7881 -
McLEOD CLARENDON
803-433-3000

 Find a Doctor
Find a Doctor  Locations
Locations  Services
Services 
